Based on Figure 18-1 Which of the Following Species Do Not Belong to the Same Family
Introduction
The genus Methylobacterium contains more species than any other genera within the family Methylobacteriaceae, order Rhizobiales, and grade Alphaproteobacteria (Kelly et al., 2014). Methylobacterium species are Gram-negative, rod-shaped leaner. The genus was first proposed by Patt et al. (1976) with Methylobacterium organophilum as the type species. The genus Methylobacterium was first emended to include facultative methylotrophs that have the power to grow on marsh gas or methanol as the source of carbon and free energy, in addition to sugars and organic acids (Patt et al., 1976). Some other taxonomic report classified all other previously known pink-pigmented facultative methylotrophic bacteria nether the genus Methylobacterium (Green and Bousfield, 1982). Thereafter, 11 species from the genus Methylobacterium were redefined into a new genus proposed every bit Methylorubrum, based on 16S rRNA gene sequence, multi-locus sequence analysis (MLSA), genomic, and phenotypic information (Green and Ardley, 2018).
The genus Methylobacterium consists of 45 recognized species, which are ubiquitously present in a wide diversity of habitats including air, soil, freshwater, and sediments, and can exist either in free-grade or associated with plant tissues (Gallego et al.,2005a,b; Kang et al., 2007; Veyisoglu et al., 2013; Kelly et al., 2014; Kwak et al., 2014; Chaudhry et al., 2016; Light-green and Ardley, 2018; Park et al., 2018). Methylobacterium species are involved in nitrogen fixation, phosphate solubilization, abiotic stress tolerance, institute growth promotion, and biocontrol activeness confronting institute pathogens (Madhaiyan et al., 2006; Kumar 1000. et al., 2016; Parasuraman et al., 2019; Grossi et al., 2020; Krug et al., 2020). For instance, a novel Methylobacterium sp. 2A was observed to outcome in college density of lateral roots in inoculated potato crops, even under common salt stress conditions, compared with control plants that were not inoculated with the bacteria; information technology was also plant to exhibit biocontrol action against several constitute pathogens (Grossi et al., 2020). Furthermore, genomic analysis of Methylobacterium sp. 2A revealed the presence of metabolic pathways involved in plant growth promotion, including the genes for producing an auxin, 3-indole acetic acid (Grossi et al., 2020).
In an ongoing Microbial Tracking experiment on the International Infinite Station (ISS), iv strains belonging to the family Methylobacteriaceae were isolated (Checinska Sielaff et al., 2019). Some of the Methylobacterium species that are phylogenetically related to these ISS strains have been isolated from plant sources (Kang et al., 2007; Chaudhry et al., 2016), indicating that the ISS strains might also brandish properties related to found growth promotion. The objectives of this study were to generate whole genome sequences (WGS) and define the phylogenetic novelty of the ISS Methylobacterium strains using polyphasic taxonomic analyses. The WGS generated and annotated in this study was used to predict biotechnologically useful genetic determinants.
Materials and Methods
Sample Collection and Isolation of Bacteria
Several surface samples (1 k2) were collected from the ISS during Microbial Tracking–1 flying experiments from 2015 to 2016. Sample drove, processing, and isolation of cultivable microorganisms were published elsewhere (Checinska Sielaff et al., 2019). Briefly, the polyester wipes used to collect samples and particulates associated with the sampling devices were transported to World before being disassociated into sterile phosphate-buffered saline (pH 7.four) solution and plated onto R2A agar medium (Checinska et al., 2015; Checinska Sielaff et al., 2019). The microbial cultures that were grown at 25°C for 7 days were picked from the R2A plates, purified, and stored for further analyses. Distinct colonies (north = iv) isolated from 3 unlike locations and from a high-efficiency particulate arrestance (HEPA) filter were characterized during this written report. These colonies exhibited unique coloration and differential genomic phylogeny. The blazon strain IF7SW-B2T was isolated during Flight i (March 2015) at Location #vii, the Overhead-three console surface of the Materials Science Research Rack 1, which is used for basic materials research in the microgravity environment of the ISS. The 2nd strain, IIF1SW-B5, was isolated during Flight 2 (May 2015) at Location #1, the Port panel of the Cupola. The Cupola is a small module devoted to the observation of operations outside the ISS, such as robotic activities, spacecraft approaches, and extravehicular activities. The third strain, IIF4SW-B5, was isolated during Flight 2 (May 2015) at Location #iv, the surface of the dining table. Fifty-fifty though the main function of the table was for dining, crewmembers also used the table for experimental piece of work. The fourth strain was I1-R3, isolated from the ISS HEPA filter that was returned aboard STS-134/ULF6 in May 2011 and archived equally reported earlier (Checinska et al., 2015).
Dna Extraction and Whole Genome Sequencing Analysis
A biomass of approximately i μg wet weight was nerveless for Deoxyribonucleic acid extraction from each strain after growing on R2A medium at 25°C for iii days. Full nucleic acrid extraction was carried out using ZymoBIOMICS 96 MagBead DNA kit (lysis tubes) (Zymo Research, United States) after dewdrop beating with a Bertin Precellys homogenizer. This was followed by library preparation using the Illumina Nextera Flex Protocol every bit per Illumina document number 1000000025416 v07. The initial amount of DNA for library preparation was quantified, and v to 12 cycles of polymerase chain reaction (PCR) amplification were carried out to normalize the output depending on the input DNA concentration. The amplified genomic Dna fragments were indexed and pooled in 384-plex configuration. Whole-genome shotgun sequencing was performed on a NovaSeq 6000 S4 flowcell PE 2 × 150 platform with a paired-end module. The data were filtered with NGS QC Toolkit v2.iii (Patel and Jain, 2012) for high-quality (HQ) vector- and adaptor-gratuitous reads for genome assembly (cutoff read length for HQ, 80%; cutoff quality score, xx). The number of filtered reads obtained were used for assembly with SPAdes 3.14.0 (Bankevich et al., 2012) genome assembler (m-mer size- 32 to 72 bases) using default parameters. The genome was annotated using the National Center for Biotechnology Information (NCBI) Prokaryotic Genome Annotation Pipeline 4.eleven (Tatusova et al., 2016; Haft et al., 2018). In add-on, functional notation of genome and seed categories were assigned to the genome by implementing the Rapid Annotations using Subsystems Engineering (RAST) tool (Aziz et al., 2008).
Genomes of all other strains used in this report were downloaded from NCBI, and the genomic relatedness of ISS strains was identified based on boilerplate nucleotide identity (ANI; FastANI) calculations (Jain et al., 2018) and digital Dna-DNA hybridization (dDDH) analysis (Meier-Kolthoff et al., 2013). FastANI was run on all the genomes using the default parameters: Mashmap identity cutoff I 0 = lxxx%, non-overlapping fragments of size l = 3Kb, and minimum count of reciprocal mappings τ = 50.
Phylogenetic Assay
Phylogenetic analysis was carried out based on 16S rRNA factor sequencing, and MLSA using vi housekeeping genes: ATP synthase F1 beta subunit (atpD), DNA strand exchange and recombination gene (recA), chaperone cistron (dnaK), Dna-directed RNA polymerase subunit beta (rpoB), glutamine synthetase type I (glnI), and DNA gyrase subunit B (gyrB), for differentiating Methylobacterium species (Greenish and Ardley, 2018). The 16S rRNA gene sequences of type strains of all 45 Methylobacterium species were included in the phylogenetic analysis. In addition, representative species of genus Methylorubrum, Enterovirga, Microvirga, and Neomegalonema from family Methylobacteriaceae, Rhizobium from social club Rhizobiales, Caulobacter from lodge Caulobacterales, in class Alphaproteobacteria were included. Pseudomonas aeruginosa was selected every bit the outgroup.
The 16S rRNA gene sequences of all strains were retrieved from NCBI except for the four ISS strains, which were recovered from their respective WGS. Phylogenetic assay based on housekeeping genes and MLSA was carried out with type strains of 24 Methylobacterium species and representative species of other genera. All the gene sequences were retrieved from the genome sequences using RAST v2.01 (Aziz et al., 2008; Overbeek et al., 2014; Brettin et al., 2015). The individual gene sequences for all strains were aligned separately using ClustalW, so the maximum likelihood tree was generated using MEGA vii.0.26 (Kumar South. et al., 2016). For MLSA, six housekeeping gene sequences for each strain were concatenated manually and aligned using ClustalW, and then the maximum likelihood tree was generated using MEGA 7.0.26 (Kumar South. et al., 2016).
The genome-based tree for the Methylobacterium species, including ISS strains and representative species of other genus with available WGS, was constructed using GToTree (Lee, 2019). This tool takes the complete/typhoon genomes as input and creates a phylogenomic tree based on the prespecified single-copy gene set using a hidden Markov model (HMM); the tool currently has ii,044 unique HMM fix as identifiers to encompass all three domains of microbial life.
Phenotypic Label of ISS Strains
Phenotypic characterization was performed according to standard protocols (Jones, 1981). Growth of the ISS strains at different temperatures (7, 25, 30, 37, and 45°C) was assessed after incubation on nutrient agar (Sigma, Usa) for 7 days. Growth at different pH (iv.0–ten.0 at intervals of 1.0) was assessed after incubation in food goop (Sigma, United states of america) at 30°C for 7 days. The pH of the food medium was adjusted using citrate/NaH2POiv buffer (pH 4.0–v.0), phosphate buffer (pH 6.0–8.0), and tris buffer (pH 9.0–10.0) (Kim et al., 2019). Salt tolerance was tested by streaking the strains on R2A supplemented with NaCl (0–10% at intervals of 1%) and incubating the plates at 30°C for 7 days. Move was assessed via the "hanging drop" method by observing the culture nether a lite microscope (Tindall et al., 2007). Catalase activeness was tested by calculation 3% hydrogen peroxide to culture grown on R2A at thirty°C for 7 days, and effervescence was monitored (Tindall et al., 2007). An oxidase examination was carried out in a filter newspaper soaked with the substrate tetramethyl-p-phenylenediamine dihydrochloride, and coloration was documented (Jurtshuk Jr. and McQuitty, 1976). All other physiological and biochemical tests were carried out using API 20 NE, API 50 CH, and API ZYM kits as per manufacturer'southward procedures (bioMérieux, France).
Chemotaxonomic Analysis
All strains grown in the R2A goop were harvested when growth of the cultures reached around seventy% of the maximal optical density (exponential growth phase), and then the cultures were used for analyses of cellular fatty acids, polar lipids, and quinones, which were carried out as described previously (Ramaprasad et al., 2015). Briefly, for cellular fat acids assay, 40 mg of bacterial cell pellet from each strain was subjected to a series of four unlike reagents followed by saponification and methylation of fatty acids, thus enabling their cleavage from lipids. The fatty acid methyl esters (FAME) thus obtained were analyzed past a gas chromatograph equipped with Sherlock MIS software (Microbial ID; MIDI half-dozen.0 version; Agilent: 6850)2. The peaks obtained were then labeled, and the equivalent chain length (ECL) values were computed past the Sherlock software.
The polar lipids contour was analyzed by extracting cells with methanol-chloroform-saline (2:i:0.8, five/v/five) from 1 one thousand of freeze-dried bacterial cells. Separation of lipids was performed by two-dimensional chromatography on a silica gel sparse-layer chromatography plate (Kieselgel threescore F254; Merck) using chloroform-methanol-water (75:32:4, v/v/5) in the first dimension and chloroform–methanol–acetic acid–water (86:16:15:4, v/v/v/v) in the 2nd dimension. The full polar lipids profile was detected by spraying with vi% ethanolic molybdophosphoric acrid. The respiratory isoprenoid quinone was extracted with a chloroform-methanol mixture (2:1, five/v), evaporated under vacuum, re-extracted with acetone, and analyzed using loftier-performance lipid chromatography as per established methods (Ramaprasad et al., 2018).
Results and Discussion
This study reports the isolation and identification of four strains belonging to the family unit Methylobacteriaceae, nerveless from different locations on the ISS. Three of the strains, referred to as IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5, were identified based on the traditional and genomic taxonomic approaches. The fourth strain, which was isolated from a HEPA filter and referred to equally I1-R3, was identified based on genomic analyses simply.
Phylogenetic Analysis of Novel ISS Strains
To confirm that iii of the ISS strains (IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5) belong to a novel species, their phylogenetic affiliations were analyzed with other species belonging to the genus Methylobacterium. The sequence similarity of these three ISS strains with validly described Methylobacterium species was <99.four% for 16S rRNA gene (Supplementary Table 1) and <97.3% for gyrB gene with the closest existence K. indicum SE2.11T. Phylogenetic analysis of these three ISS strains was carried out past amalgam a maximum likelihood tree based on 16S rRNA (Effigy 1), gyrB (Figure 2), atpD (Supplementary Figure ane), recA (Supplementary Figure 2), dnaK (Supplementary Figure 3), rpoB (Supplementary Figure 4), and glnI (Supplementary Effigy 5) gene sequences. In addition, MLSA was carried out by concatenating the six housekeeping genes manually (Figure 3). In addition, a phylogenetic tree based on WGS was generated (Figure 4). The phylogenetic trees constructed based on all these genes, MLSA, and WGS showed that these 3 ISS strains (IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5) are amassed together and in the aforementioned clade with One thousand. indicum SE2.11T. The 16S rRNA gene-sequencing, housekeeping cistron-based analyses, MLSA, and genome-based tree farther supported the concept that these three ISS strains belong to the same species but are closely related to M. indicum. In add-on, the identity of the ISS strain I1-R3 was further confirmed to be M. rhodesianum based on its 16S rRNA cistron (Figure 1) and gyrB (Figure two) phylogenetic amalgamation to the type strain M. rhodesianum DSM 5687T.

Effigy 1. Maximum likelihood phylogenetic tree based on 16S rRNA gene sequences shows the relationship of Methylobacterium ajmalii sp. nov. with members of the family Methylobacteriaceae. Bootstrap values from 1,000 replications are shown at branch points. Bar, 0.02 substitution per site.
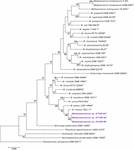
Effigy 2. Maximum likelihood phylogenetic tree, based on DNA gyrase cistron (gyrB) sequences, showing the phylogenetic relationship of Methylobacterium ajmalii sp. nov. with members of the family Methylobacteriaceae. Bootstrap values from 1,000 replications are shown at co-operative points. Bar, 0.05 exchange per site.

Figure 3. Maximum likelihood phylogenetic tree, based on six cistron sequences (atpD, recA, dnaK, rpoB, glnI, and gyrB) concatenated manually, showing the phylogenetic human relationship of Methylobacterium ajmalii sp. november. with members of the family Methylobacteriaceae. Bootstrap values from 1,000 replications are shown at branch points. Bar, 0.05 substitution per site.
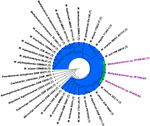
Figure 4. Genome-based phylogenetic tree showing the phylogenetic relationship of Methylobacterium ajmalii sp. nov. with members of the family Methylobacteriaceae.
Whole Genome Sequence–Based Phylogenetic Analysis
The genomes of the 4 isolated ISS strains were sequenced, with their draft genome assembled and annotated. The results are summarized in Table 1. The genome varied in size from 6.1 to half dozen.8 Mbp with GC content between 68 and 71%, similar to other members of the family unit Methylobacteriaceae.

Table ane. Summary of the draft whole-genome sequences of four strains belonging to the family unit Methylobacteriaceae, isolated from the ISS.
Due to higher sequence similarities of iii ISS strains with Yard. indicum SE2.elevenT (99.four% for 16S rRNA gene and 97.3% for gyrB gene), the typhoon genomes of 3 ISS strains were subjected to ANI and dDDH assay with other species belonging to family Methylobacteriaceae (Table 2). The ANI indices of three ISS strains (IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5) with K. indicum SE2.11T were 92.7 to 93%, and dDDH values were 45.8 to 46.four%. The ANI and dDDH values obtained for three ISS strains with other Methylobacterium species were beneath the threshold of 95% ANI (Yoon et al., 2017) and 70% dDDH values (Auch et al., 2010), which were established for prokaryotic species delineation. This suggested that these three ISS strains are novel species of the genus Methylobacterium. These 3 ISS strains exhibited ANI and dDDH values around 99–100% with each other, indicating that they vest to the same species. The entire genomes of these iii ISS strains, M. indicum SE2.11T, and M. platani PMB02T were aligned to discover their divergence and similarity using the MUMmer 3.0 system (Kurtz et al., 2004). Every bit shown in Supplementary Effigy vi, genomes of these three ISS strains aligned perfectly, while the closest genomes of Chiliad. indicum and M. platani exhibited departure with the ISS blazon strain IF7SW-B2T. Since these 3 ISS strains were isolated at dissimilar fourth dimension periods and from various locations, their persistence in the ISS environment and ecological significance in the closed systems warrant farther study.
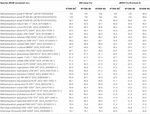
Table 2. Genomic analyses of Methylobacterium ajmalii in comparison to other species of the family Methylobacteriaceae.
The 4th strain I1-R3 was identified equally M. rhodesianum based on highly similar 16S rRNA (99.9%), gyrB (100%), ANI (98.9%), and dDDH (91.vi%) genomic parameters with M. rhodesianum DSM 5687T. The pigmentation of the strain I1-R3 (lite pink) was also unlike from the novel ISS Methylobacterium strains (carmine pink). The ANI and dDDH values betwixt I1-R3 and the 3 novel ISS Methylobacterium strains were ∼82% and 24%, respectively. Hence, genomic and morphological analyses confirmed the phylogenetic affiliation of strain I1-R3 every bit One thousand. rhodesianum. In this communication, phylogenetic affiliations of only IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5 strains were presented.
Phenotypic Label of Novel ISS Strains
The minimal information about the ISS strain genome characteristics are given in Supplementary Tabular array 2. The differential phenotypic characteristics of IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5 are listed in Table 3, in comparing with other related Methylobacterium species. Iii strains belonging to Methylobacterium sp. nov. are ruby pink–pigmented, Gram-stain-negative, catalase-positive, oxidase-positive, motile, and rod-shaped. These strains grew well on food agar and R2A. These iii strains grew optimally at temperatures between 25 and 30°C, were viable only at pH vi.0 to eight.0, and exhibited poor tolerance to salt (0 to 1%). Absence of growth was observed when grown at 7, 37, and 45°C. These strains were positive for assimilation of L-arabinose, D-glucose, maltose, D-mannitol, D-mannose, malic acrid, potassium gluconate, and trisodium citrate. These strains also exhibited esterase lipase and trypsin enzymatic activities. The consummate results of phenotypic characteristics determined using API xx NE, API ZYM, and API 50 CH are detailed in Supplementary Tables iii-v, respectively. The majority of the phenotypic characteristics of the ISS strains were similar to other Methylobacterium species. Phenotypically, these iii ISS strains were unlike from the closest genomic relative M. indicum in assimilating glucose, malic acrid, maltose, mannitol, potassium gluconate, and trisodium citrate. Furthermore, dissimilar M. indicum, these ISS strains did not exhibit growth at pH 5.0. In comparison to other Methylobacterium species, 1000. aquaticum and Thousand. terrae exhibit similar carbon substrate utilization and enzyme production profiles. However, malic acid was assimilated past these ISS strains but not by M. aquaticum. Maltose was also utilized by these ISS strains but not by Grand. terrae cells.
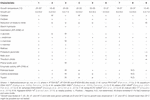
Tabular array 3. Differential phenotypic characteristics of Methylobacterium ajmalii and related species of genus Methylobacterium.
The master phenotypic characteristics of the ISS strains IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5 were in accordance with the description of the genus Methylobacterium, with the most of import being reddish pink pigmentation (Green and Bousfield, 1982). The optimum growth conditions (temperature, pH, salt tolerance) of the ISS Methylobacterium strains were similar to other members belonging to the genus Methylobacterium. Also, these three ISS strains shared the properties of exhibiting catalase activity and motility with other Methylobacterium species. All the same, the three novel ISS Methylobacterium strains differed from other members of the genus Methylobacterium in some of the phenotypic characteristics, as shown in Table 3. For instance, they exhibited properties like assimilation of certain sugars, which was absent-minded in some of the Methylobacterium species. They besides did non show cystine arylamidase activeness equally opposed to several related Methylobacterium species.
Chemotaxonomic Characterization of Novel ISS Strains
The FAME profiling of three ISS strains and other related Methylobacterium species are given in Table four. The major fatty acids in these ISS strains were Ceighteen : 1 ω7c and/or Ceighteen : i ω6c (Sum in Characteristic eight; 82 to 85%) with pocket-sized amounts of C18 : 0 3-OH, C16 : 0, C17 : 0, Sum in Feature iii, Sum in Characteristic 2, C18 : 0 and C12 : 0, and traces of Cxi : 0, C13 : 0, and C14 : 0 . The fatty acids, C18 : 1 ω7c and/or C18 : ane ω6c, were observed to exist dominant in these ISS strains, like to other species. Nonetheless, complete FAME profiles were not consistent among Methylobacterium species and some significant differences in the proportions of certain fatty acids were observed (Table 4). The notable deviation in the FAME profile was the lower abundance of Cxviii : 1 ω7c in M. indicum (46%) when compared with these ISS strains (82 to 85%).
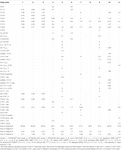
Table 4. Percentage of total cellular fatty acids from Methylobacterium ajmalii and related species of genus Methylobacterium.
These ISS strains contained Q-10 equally the major respiratory isoprenoid quinone, which is mutual in members of the genus Methylobacterium. The polar lipids present in these three strains were diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), phosphatidyl choline (PC), phosphatidyl-ethanolamine (PE), and an unidentified lipid (Supplementary Figure 7). The total polar lipid profile of these ISS strains was consistent with their close relatives, predominated with phospholipids, DPG, PG, and PE. Furthermore, the chemotaxonomic data together with the results of the genomic and phylogenetic analysis support the affiliation of strains IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5 to the genus Methylobacterium.
Functional Characteristics of the Novel ISS Strain
The genome of the ISS strain IF7SW-B2T, type strain, was annotated and analyzed to decide biotechnologically important genetic determinants. The whole genome and annotation analysis predicted a total of 6,531 genes in the assembled draft genome. Among these, i,430 brutal into various RAST categories, contributing to two,067 predicted features described in Table five. All the ane,430 feature and subsystems have been documented in Supplementary Data one. A major fraction of the annotated genes was composed of amino acids and derivatives (408), carbohydrate metabolism (246), poly peptide metabolism (198), genes associated with cofactors, vitamins, prosthetic groups, pigments metabolism (190), and respiration (151) (Tabular array v). Genes responsible for motility and chemotaxis (95), metabolism of aromatic compounds (47), and stress response (72) were also observed.

Tabular array 5. Genes belonging to different functional categories based on annotation generated using RAST for Methylobacterium ajmalii IF7SW-B2T.
Based on the genome annotation, genes for nitrogen metabolism were predicted in the genome of the ISS strain IF7SW-B2T. Most of the subsystem features aligned with the ammonia assimilation pathway (eleven genes), which is a preferred nitrogen source for the bacteria (Leigh and Dodsworth, 2007). In improver, metabolic factors similar to high-affinity phosphate transporter and command of Pho regulon were besides identified in the ISS strain IF7SW-B2T (Wanner, 1993, 1996). Interestingly, a higher number of stress tolerance genes, specially the oxidative stress response factors, were observed in the ISS strain IF7SW-B2T when compared with other novel species isolated from the ISS; Methylobacterium sp. IF7SW-B2T exhibited 58 features, whereas 36 features were identified in Solibacillus kalamii (Seuylemezian et al., 2017) and 18 features were identified in Kalamiella piersonii (Singh et al., 2019). The results obtained agree with the previous reports that showed altered regulation of the stress response factors in microorganisms, in the presence of microgravity conditions (Orsini et al., 2017; Aunins et al., 2018). Further studies on the role of oxidative stress in species option are warranted. The WGS associates of these three ISS strains reported hither volition enable the comparative genomic label of ISS isolates with Globe counterparts in future studies. This will further aid in the identification of genetic determinants that might potentially be responsible for promoting institute growth nether microgravity atmospheric condition and contribute to the development of self-sustainable plant crops for long-term infinite missions in future.
Genes Essential for Interaction With Plants in the ISS Strain
A thorough genomic assay of the ISS strain IF7SW-B2T revealed the presence of genes that accept been involved in promoting institute growth. The isopentenyl tRNA transferase (miaA) essential for cytokinin production reported in Thousand. aquaticum strain 22A (Tani et al., 2015) was as well found in genome of the ISS strain IF7SW-B2T with loftier similarity. The product of the miaA gene was reported to be responsible for isopentenylation of a specific adenine in some tRNAs and confirmed the secretion of zeatin originated from tRNA in M. extorquens (Koenig et al., 2002). Furthermore, multiple components of the cobalamin synthesis pathway, such as cobalamin biosynthesis poly peptide BluB, L-threonine 3-O-phosphate decarboxylase (EC 4.i.one.81), adenosylcobinamide-phosphate guanylyltransferase (EC 2.7.7.62), cobyric acid synthase (EC 6.three.5.10), nicotinate-nucleotide—dimethylbenzimidazole phosphoribosyltransferase (EC 2.four.two.21), adenosylcobinamide-phosphate synthase (EC 6.3.i.10), cob(I)alamin adenosyltransferase. (EC two.5.1.17), cobalamin synthase (EC 2.vii.8.26), and adenosylcobinamide kinase (EC 2.7.1.156), were identified in genome of the ISS strain IF7SW-B2T. The metabolic pathway for cobalamin synthesis predicted in the ISS strain is presented (Supplementary Effigy 8). Supporting this prediction, previous study also reported that Methylobacterium strains harbor genes involved in the production of a variety of vitamins, such equally cobalamin, biotin, thiamin, and riboflavin, indicating the potential of methylobacteria promoting algal growth (Krug et al., 2020). In improver, genes associated with siderophore product, i.east., ferric siderophore transport system, biopolymer transport protein ExbB, and multiple flagellar proteins, were identified in genome of the ISS strain IF7SW-B2T and are listed in the Supplementary Figure 8. Genes involved in iron acquisition and metabolism in which microalgae benefit from bacterial siderophores take been reported previously in Methylobacterium spp. (Krug et al., 2020). In the "carbon for fe mutualism" concept, algae assimilated iron complexed in bacterial siderophores and in render provided essential dissolved organic matter for the bacteria (Amin et al., 2015). Like studies are warranted to confirm the plant-growth promoting activities in the IF7SW-B2T ISS strain.
In summary, the phylogenetic and genetic distinctiveness and differential phenotypic properties were sufficient to categorize these three ISS strains every bit members of a species distinct from other recognized Methylobacterium species. Therefore, on the ground of the data presented, strains IF7SW-B2T, IIF1SW-B5, and IIF4SW-B5 stand for a novel species of the genus Methylobacterium, for which the proper name Methylobacterium ajmalii sp. nov. is proposed. The type strain is IF7SW-B2T (NRRL B-65601T and LMG 32165T).
Description of Methylobacterium ajmalii sp. nov.
Methylobacterium ajmalii (aj.ma'li.i. N.50. gen. n. ajmalii named after Ajmal Khan, a renowned Indian scientist on biodiversity). Cells are Gram-stain-negative, aerobic, and motile rods showing oxidase- and catalase-positive reactions. Cells are one.6–1.viii μm wide and 2.two–3.2 μm long. Colonies on R2A agar are reddish pink–pigmented, circular, convex, and smooth, with a diameter of approximately 0.6–1.0 mm afterwards three days of incubation on R2A agar. Growth occurs at 25–30°C (optimum, xxx°C), at pH six.0–viii.0 (optimum, pH 7.0) and in the presence of 0–1.0% (west/v) NaCl (optimum, 0%). In API ZYM tests, the strain is positive for Alkaline phosphatase, Esterase (C4), Esterase lipase (C8), Leucine arylamidase, Trypsin, Acid phosphatase, and Naphthol-Every bit-BI-phosphohydrolase, but negative for other enzyme activities. Cells apply Adipic acid, D-glucose, D-maltose, D-mannitol, D-mannose, L-arabinose, Malic acid, N-acetyl-glucosamine, Potassium gluconate, and Trisodium citrate for growth, but not other substrates in API 20NE. Cells are capable of weakly fermenting inulin and D-melezitose as observed in API 50 CH. Ubiquinone Q-10 is the predominant respiratory isoprenoid quinone. The major fat acid is summed feature 8 (comprising C18:1 ω7c and/or C18:1 ω6c). The major polar lipids are diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylcholine, and phosphatidylglycerol. The genomic DNA G + C content of the blazon strain is 71.07 mol%.
The type strain IF7SW-B2T is isolated from the International Space Station.
Information Availability Statement
The 16S rRNA factor sequences of Methylobacterium sp. IIF1SW-B5, and Methylobacterium sp. IIF4SW-B5 are submitted under accretion numbers KY218843 and KY218865, respectively. The WGS and the raw data deposited under BioProject accession number PRJNA634337. The WGS accession numbers are mentioned in Table 1. The WGS was likewise deposited in GeneLab under GeneLab dataset (GLDS-300; https://genelab-data.ndc.nasa.gov/genelab/accession/GLDS339 300). The version described in this paper is the kickoff version.
Author Contributions
KV and NKS conceived and designed the experiments. SB, VE, and NKS performed the experiments. NKS analyzed the genomic data inclusive of de novo assemblies and verification, scaffold quality cess, and annotation and generation of the whole genome and protein level alignment for positional description of organism in the tree of life. SB independently verified the genome associates, generated alignments for all gene copse in the manuscript, and manually curated the tree images. KV and NKS isolated the type strain, and NKS carried out the phenotypic assays and biochemical characterization. KV compiled the contribution of write-ups from all authors associated with phenotype, NKS generated genotype and tables, and SB generated phylogenetic trees and figures. VE conducted the SB generated chemotaxonomic analysis. All authors read and approved the final manuscript. CEM generated the genomic library and sequenced the genomes of all strains. CCCW and ARP reviewed the manuscript.
Funding
The inquiry described in this manuscript was funded by a 2012 Space Biology NNH12ZTT001N Grant No. 19-12829-26 under Job Order NNN13D111T awarded to KV, and NASA's 2018 Infinite Biological science (ROSBio) NNH18ZTT001N-FG App B: Flying and Ground Space Biology Research Grant No. 80NSSC19K1501 awarded to CCCW.
Disharmonize of Interest
The author(s) declare that there are no conflicts of interest. This manuscript was prepared every bit an account of work sponsored by NASA, an bureau of the Us Government. The US Government, NASA, California Institute of Technology, Jet Propulsion Laboratory, and their employees make no warranty, expressed or implied, or presume any liability or responsibility for the accuracy, completeness, or usefulness of information, apparatus, product, or process disclosed in this manuscript, or represents that its use would not infringe upon privately held rights. The use of, and references to whatsoever commercial production, process, or service does non necessarily constitute or imply endorsement, recommendation, or favoring by the U.Southward. Government, NASA, California Constitute of Technology, or Jet Propulsion Laboratory. Views and opinions presented herein by the authors of this manuscript exercise not necessarily reverberate those of the U.S. Government, NASA, California Institute of Technology, or Jet Propulsion Laboratory, and shall not be used for advertisements or product endorsements.
Acknowledgments
The research described in this manuscript was performed at the Jet Propulsion Laboratory, California Found of Technology under a contract with NASA and University of Southern California. Nosotros would similar to thank Aleksandra Checinska-Sielaff for isolating the strain. Nosotros thank astronauts Captain Terry Virts for collecting samples aboard the ISS and the Implementation Team at NASA Ames Research Eye (Fathi Karouia) for coordinating this effort. We also thank Ryan Kemp (Zymo Corp.) for extracting DNA and Dan Butler (Cornell Medicine) for performing shotgun sequencing using the NovaSeq platform. We besides acknowledged the Jet Propulsion Laboratory supercomputing facility staff, notably Narendra J. Patel (Jimmy) and Edward Villanueva, for their continuous support in providing the best possible infrastructure for BIG-Information analysis. ©2021 California Institute of Applied science. Government sponsorship acknowledged.
Supplementary Material
The Supplementary Material for this article can be institute online at: https://www.frontiersin.org/articles/x.3389/fmicb.2021.639396/full#supplementary-material
Footnotes
- ^ https://rast.nmpdr.org/
- ^ http://midi-inc.com
References
Amin, S. A., Hmelo, L. R., van Tol, H. Chiliad., Durham, B. P., Carlson, 50. T., Heal, K. R., et al. (2015). Interaction and signalling betwixt a cosmopolitan phytoplankton and associated leaner. Nature 522, 98–101. doi: x.1038/nature14488
PubMed Abstract | CrossRef Full Text | Google Scholar
Auch, A. F., von January, Thou., Klenk, H.-P., and Göker, M. (2010). Digital Dna-Deoxyribonucleic acid hybridization for microbial species delineation past ways of genome-to-genome sequence comparison. Standards Genom. Sci. 2, 117–134. doi: 10.4056/sigs.531120
PubMed Abstruse | CrossRef Full Text | Google Scholar
Aunins, T. R., Erickson, Yard. E., Prasad, North., Levy, Southward. E., Jones, A., Shrestha, Southward., et al. (2018). Spaceflight modifies Escherichia coli cistron expression in response to antibiotic exposure and reveals role of oxidative stress response. Front. Microbiol. 9:310. doi: 10.3389/fmicb.2018.00310
PubMed Abstract | CrossRef Full Text | Google Scholar
Aziz, R. G., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genom. nine:75. doi: 10.1186/1471-2164-9-75
PubMed Abstract | CrossRef Total Text | Google Scholar
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. South., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. xix, 455–477. doi: x.1089/cmb.2012.0021
PubMed Abstract | CrossRef Full Text | Google Scholar
Brettin, T., Davis, J. J., Disz, T., Edwards, R. A., Gerdes, S., Olsen, G. J., et al. (2015). RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. v:8365.
Google Scholar
Chaudhry, Five., Baindara, P., Pal, V. K., Chawla, North., Patil, P. B., and Korpole, S. (2016). Methylobacterium indicum sp. nov., a facultative methylotrophic bacterium isolated from rice seed. Syst. Appl. Microbiol. 39, 25–32. doi: 10.1016/j.syapm.2015.12.006
PubMed Abstruse | CrossRef Full Text | Google Scholar
Checinska, A., Probst, A. J., Vaishampayan, P., White, J. R., Kumar, D., Stepanov, V. Yard., et al. (2015). Microbiomes of the dust particles collected from the international infinite station and spacecraft assembly facilities. Microbiome 3:50.
Google Scholar
Checinska Sielaff, A., Urbaniak, C., Mohan, G. B. Thou., Stepanov, Five. One thousand., Tran, Q., Forest, J. M., et al. (2019). Characterization of the full and viable bacterial and fungal communities associated with the International Infinite Station surfaces. Microbiome 7:50.
Google Scholar
Gallego, V., Garcia, M. T., and Ventosa, A. (2005a). Methylobacterium hispanicum sp. november. and Methylobacterium aquaticum sp. nov., isolated from drinking h2o. Int. J. Syst. Evol. Microbiol. 55, 281–287. doi: x.1099/ijs.0.63319-0
PubMed Abstract | CrossRef Total Text | Google Scholar
Gallego, 5., Garcia, M. T., and Ventosa, A. (2005b). Methylobacterium variabile sp. nov., a methylotrophic bacterium isolated from an aquatic environment. Int. J. Syst. Evol. Microbiol. 55, 1429–1433. doi: 10.1099/ijs.0.63597-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Green, P. N., and Ardley, J. K. (2018). Review of the genus Methylobacterium and closely related organisms: a proposal that some Methylobacterium species be reclassified into a new genus. methylorubrum gen. november. Int. J. Syst. Evol. Microbiol. 68, 2727–2748. doi: x.1099/ijsem.0.002856
PubMed Abstract | CrossRef Full Text | Google Scholar
Green, P. N., and Bousfield, I. J. (1982). A taxonomic study of some Gram-negative facultatively methylotrophic bacteria. Microbiology 128, 623–638. doi: ten.1099/00221287-128-3-623
CrossRef Total Text | Google Scholar
Grossi, C. E. M., Fantino, Eastward., Serral, F., Zawoznik, K. S., Fernandez, Do Porto, D. A., et al. (2020). Methylobacterium sp. 2A is a found growth-promoting rhizobacteria that has the potential to improve potato crop yield under adverse weather. Front. Plant Sci. 11:71. doi: 10.3389/fpls.2020.00071
PubMed Abstract | CrossRef Total Text | Google Scholar
Haft, D. H., DiCuccio, M., Badretdin, A., Brover, V., Chetvernin, V., O'Neill, K., et al. (2018). RefSeq: an update on prokaryotic genome notation and curation. Nucleic Acids Res. 46, D851–D860.
Google Scholar
Jain, C., Rodriguez, R. L., Phillippy, A. M., Konstantinidis, M. T., and Aluru, S. (2018). Loftier throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9:5114.
Google Scholar
Jurtshuk, P. Jr., and McQuitty, D. Northward. (1976). Use of a quantitative oxidase test for characterizing oxidative metabolism in bacteria. Appl. Environ. Microbiol. 31, 668–679. doi: 10.1128/aem.31.5.668-679.1976
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kang, Y. Due south., Kim, J., Shin, H. D., Nam, Y. D., Bae, J. W., Jeon, C. O., et al. (2007). Methylobacterium platani sp. nov., isolated from a leaf of the tree Platanus orientalis. Int. J. Syst. Evol. Microbiol. 57, 2849–2853. doi: 10.1099/ijs.0.65262-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Kelly, D. P., McDonald, I. R., and Wood, A. P. (2014). "The family methylobacteriaceae," in The Prokaryotes- Alphaproteobacteria and Betaproteobacteria, eds Due east. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson, (Berlin: Springer), 313–340. doi: 10.1007/978-3-642-30197-1_256
CrossRef Full Text | Google Scholar
Kim, J., Chhetri, Yard., Kim, I., Kim, H., Kim, M. 1000., and Seo, T. (2019). Methylobacterium terrae sp. nov., a radiations-resistant bacterium isolated from gamma ray-irradiated soil. J. Microbiol. 57, 959–966. doi: ten.1007/s12275-019-9007-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Koenig, R. 50., Morris, R. O., and Polacco, J. C. (2002). tRNA is the source of depression-level trans-zeatin production in Methylobacterium spp. J. Bacteriol. 184, 1832–1842. doi: ten.1128/jb.184.7.1832-1842.2002
PubMed Abstract | CrossRef Total Text | Google Scholar
Krug, Fifty., Morauf, C., Donat, C., Muller, H., Cernava, T., and Berg, M. (2020). Found growth-promoting methylobacteria selectively increase the biomass of biotechnologically relevant microalgae. Front. Microbiol. eleven:427. doi: ten.3389/fmicb.2020.00427
PubMed Abstract | CrossRef Total Text | Google Scholar
Kumar, M., Tomar, R. S., Lade, H., and Paul, D. (2016). Methylotrophic bacteria in sustainable agriculture. World J. Microbiol. Biotechnol. 32:120.
Google Scholar
Kumar, Southward., Stecher, Thousand., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: x.1093/molbev/msw054
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kurtz, Southward., Phillippy, A., Delcher, A. L., Smoot, Thou., Shumway, G., Antonescu, C., et al. (2004). Versatile and open up software for comparing large genomes. Genome Biol. 5:R12.
Google Scholar
Kwak, Chiliad. J., Jeong, H., Madhaiyan, Chiliad., Lee, Y., Sa, T. M., Oh, T. Yard., et al. (2014). Genome information of Methylobacterium oryzae, a plant-probiotic methylotroph in the phyllosphere. PLoS One nine:e106704. doi: ten.1371/journal.pone.0106704
PubMed Abstract | CrossRef Full Text | Google Scholar
>Lee, Y., and Jeon, C. O. (2018). Methylobacterium frigidaeris sp. nov., isolated from an air conditioning system. Int. J. Syst. Evol. Microbiol. 68, 299–304. doi: 10.1099/ijsem.0.002500
PubMed Abstract | CrossRef Full Text | Google Scholar
Madhaiyan, M., Suresh Reddy, B. V., Anandham, R., Senthilkumar, M., Poonguzhali, Southward., Sundaram, Due south. P., et al. (2006). Institute growth-promoting methylobacterium induces defense responses in groundnut (Arachis hypogaea 50.) compared with rot pathogens. Curr. Microbiol. 53, 270–276. doi: ten.1007/s00284-005-0452-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P., and Goker, M. (2013). Genome sequence-based species delimitation with confidence intervals and improved altitude functions. BMC Bioinform. 14:sixty. doi: 10.1186/1471-2105-14-60
PubMed Abstract | CrossRef Full Text | Google Scholar
Orsini, S. S., Lewis, A. M., and Rice, K. C. (2017). Investigation of false microgravity effects on Streptococcus mutans physiology and global gene expression. NPJ Microgravity 3:four.
Google Scholar
Overbeek, R., Olson, R., Pusch, G. D., Olsen, 1000. J., Davis, J. J., Disz, T., et al. (2014). The seed and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 42, D206–D214.
Google Scholar
Parasuraman, P., Pattnaik, S., and Busi, S. (2019). Phyllosphere Microbiome: Functional Importance in Sustainable Agriculture, New and Future Developments in Microbial Biotechnology and Bioengineering. Amsterdam: Elsevier, 135–148.
Google Scholar
Park, C., Lee, Y. S., Park, S. Y., and Park, West. (2018). Methylobacterium currus sp. nov., isolated from a car ac arrangement. Int. J. Syst. Evol. Microbiol. 68, 3621–3626. doi: 10.1099/ijsem.0.003045
PubMed Abstract | CrossRef Full Text | Google Scholar
Patt, T. Due east., Cole, G. C., and Hanson, R. S. (1976). Methylobacterium, a new genus of facultatively methylotrophic bacteria. Int. J. Syst. Bacteriol. 26, 226–229. doi: ten.1099/00207713-26-2-226
CrossRef Full Text | Google Scholar
Ramaprasad, E. 5. V., Mahidhara, G., Sasikala, C., and Ramana, C. 5. (2018). Rhodococcus electrodiphilus sp. nov., a marine electro active actinobacterium isolated from coral reef. Int. J. Syst. Evol. Microbiol. 68, 2644–2649. doi: ten.1099/ijsem.0.002895
PubMed Abstract | CrossRef Full Text | Google Scholar
Ramaprasad, E. V. V., Sasikala, C., and Ramana, C. V. (2015). Flectobacillus rhizosphaerae sp. nov., isolated from the rhizosphere soil of Oryza sativa (L.), and emended clarification of the genus Flectobaicillus. Int. J. Syst. Evol. Microbiol. 65, 3451–3456. doi: ten.1099/ijsem.0.000432
PubMed Abstruse | CrossRef Total Text | Google Scholar
Seuylemezian, A., Singh, Northward. 1000., Vaishampayan, P., and Venkateswaran, Chiliad. (2017). Typhoon genome sequence of solibacillus kalamii, isolated from an air filter aboard the international space station. Genome Announc. 5:e00696-17.
Google Scholar
Singh, N. K., Wood, J. M., Mhatre, S. South., and Venkateswaran, K. (2019). Metagenome to phenome approach enables isolation and genomics characterization of Kalamiella piersonii gen. nov., sp. nov. from the international space station. Appl. Microbiol. Biotechnol. 103, 4483–4497.
Google Scholar
Tani, A., Ogura, Y., Hayashi, T., and Kimbara, K. (2015). Complete genome sequence of methylobacterium aquaticum strain 22a, isolated from Racomitrium japonicum moss. Genome Announc. 3:e00266-fifteen.
Google Scholar
Tatusova, T., DiCuccio, Yard., Badretdin, A., Chetvernin, V., Nawrocki, East. P., Zaslavsky, 50., et al. (2016). NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624.
Google Scholar
Tindall, B., Sikorski, J., Smibert, R., and Krieg, N. (2007). "Phenotypic characterization and the principles of comparative systematics," in Methods for General and Molecular Microbiology, eds C. Reddy, T. Beveridge, J. Breznak, G. Marzluf, T. Schmidt, and 50. Snyder, (Washington, DC: ASM Press), 330–393.
Google Scholar
Veyisoglu, A., Camas, M., Tatar, D., Guven, Chiliad., Sazak, A., and Sahin, Northward. (2013). Methylobacterium tarhaniae sp. nov., isolated from arid soil. Int. J. Syst. Evol. Microbiol. 63, 2823–2828.
Google Scholar
Wanner, B. 50. (1993). Gene regulation by phosphate in enteric leaner. J. Cell. Biochem. 51, 47–54.
Google Scholar
Wanner, B. L. (1996). "Phosphorus assimilation and control of the phosphate regulon," in Escherichia coli and Salmonella: cellular and molecular biology, ed. F. C. Neidhardt, (Washington, DC: ASM press), 1357–1381.
Google Scholar
Yoon, S. H., Ha, S. M., Lim, J., Kwon, Due south., and Chun, J. (2017). A big-calibration evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110, 1281–1286.
Google Scholar
robinsonlinto1947.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fmicb.2021.639396/full
Post a Comment for "Based on Figure 18-1 Which of the Following Species Do Not Belong to the Same Family"